missing translation for 'onlineSavingsMsg'
Learn More
Learn More
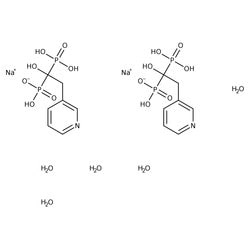
Risedronate Sodium Pharmaceutical Secondary Standard, MilliporeSigma™ Supelco™
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Supplier: Merck Emd Millipore PHR18881G
Specifications
| 329003-65-8 | |
| C7H10 NNaO7P2 · 2.5H2 O | |
| Risedronic acid monosodium salt hemi(pentahydrate) | |
| 1 g | |
| Certified Reference Material |
| These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available. | |
| MFCD01706268 | |
| Certified Reference Material | |
| 350.13 |